Mechanism of multiorgan remodelling in Systemic Sclerosis
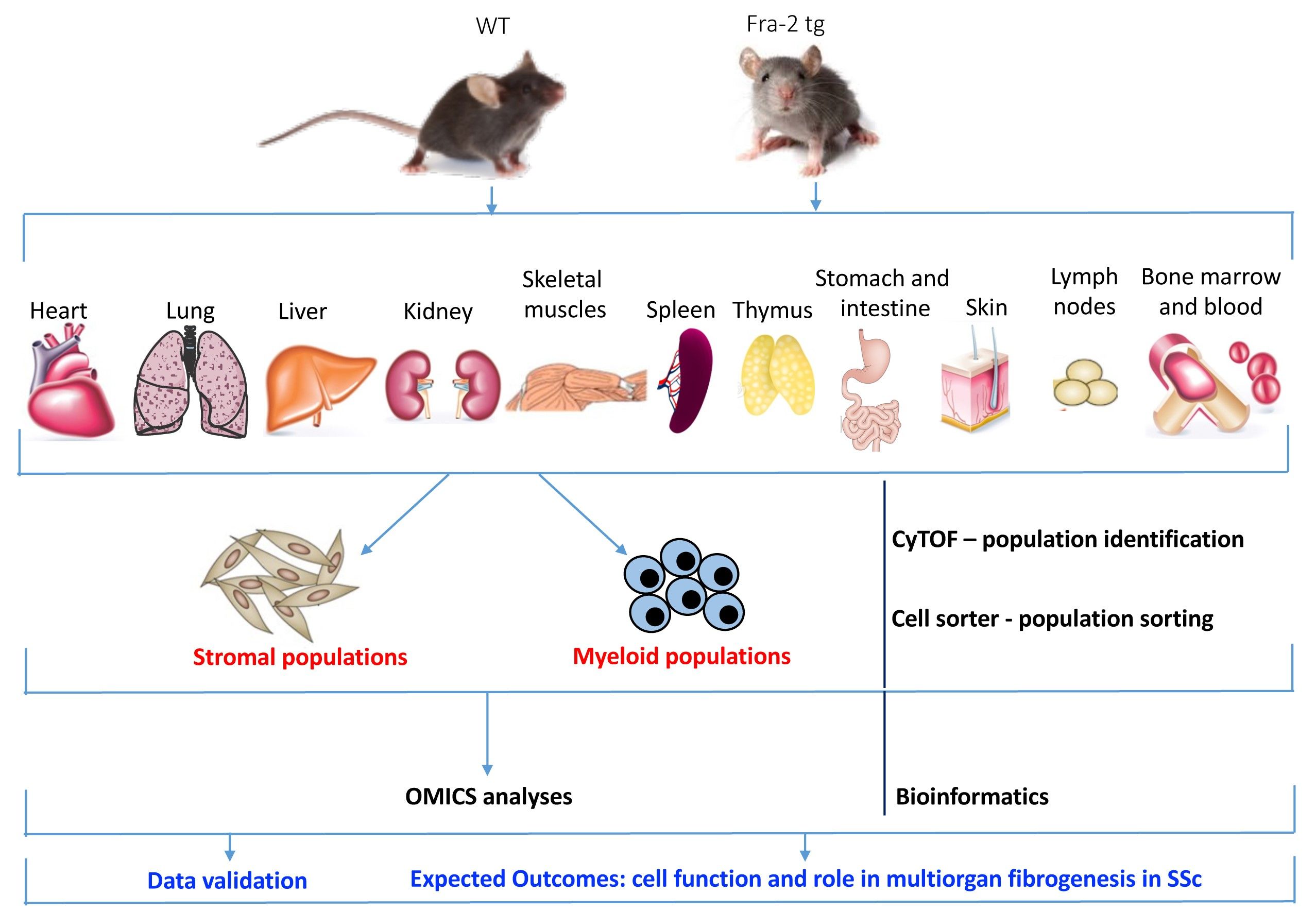
Understanding the cellular and molecular bases of multiorgan fibrogenesis leading to the fatal organ remodelling in Systemic Sclerosis (SSc) is crucial for identifying potential diagnostic and therapeutic targets. Inflammation, fibrosis and vasculopathy are the hallmarks of the rare, autoimmune disease SSc. Nevertheless, despite the high unmet clinical need, so far little is known about the etiology of SSc pathogenesis and the mechanisms leading to multiorgan dysfunction in SSc patients.
We are interested in studying the role of specific stromal cell and inflammatory myeloid cell compartments in multiorgan fibrogenesis and pathological remodelling, as well in determining the origin of pathological myofibroblasts and their cellular sources in SSc. We are particularly focusing on: i) single cell level, ii) cell-to-cell interaction during the process of multiorgan inflammation and fibrogenesis, and iii) molecular mechanisms that drive this complicated signalling orchestra.
Current projects
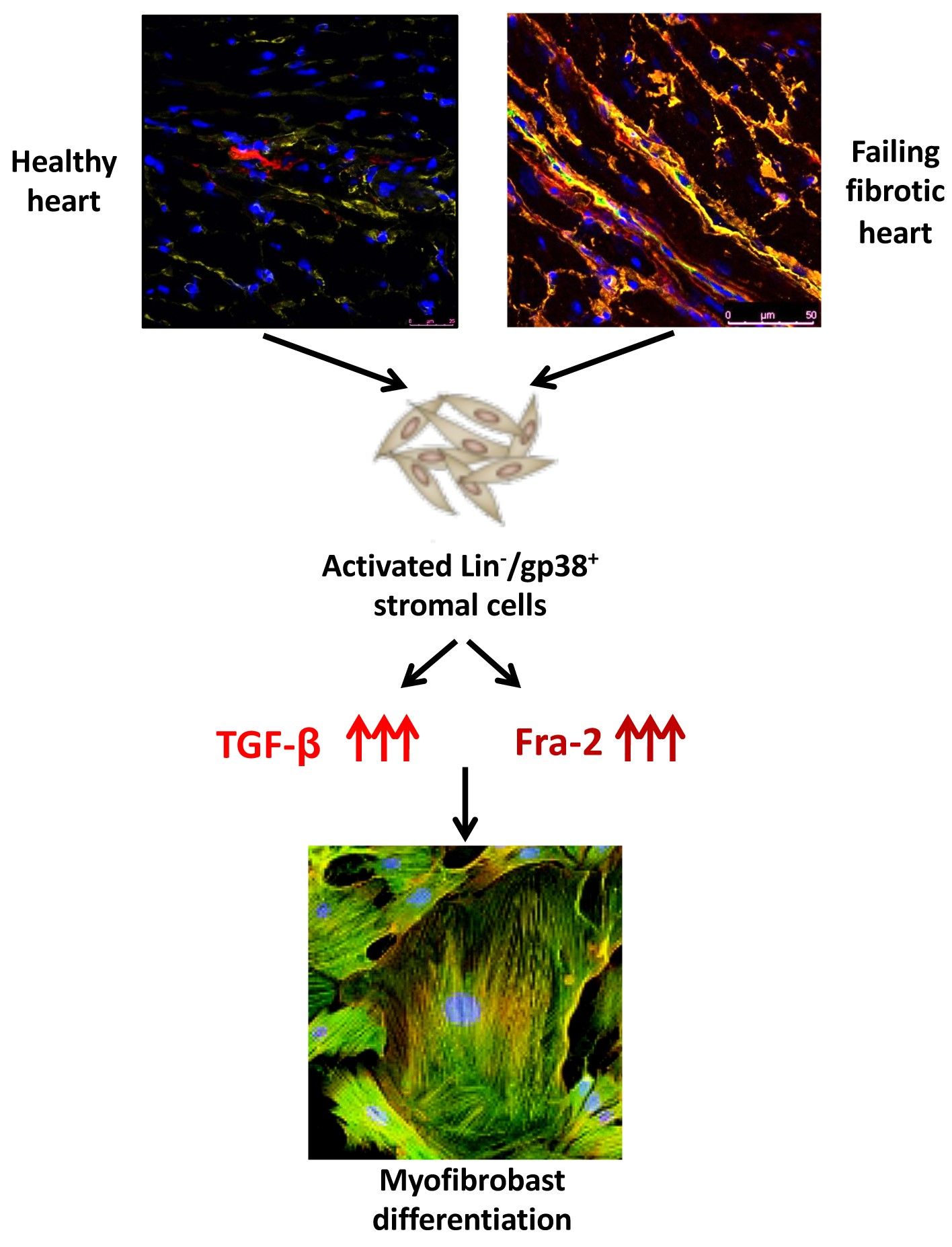
Specific stromal cell population serves as cellular sources of pathological myofibroblasts- key players in the heart remodelling in SSc. We pointed to the key role of Transforming Growth Factor (TGF)-β and Fos-related antigen (Fra)-2 in the fatal cardiac remodelling in SSc. To this aim we will focus on cell-to-cell interaction and on single cell level.
Funding
- Swiss National Science Foundation
- Novartis Stiftung
- Egli Stiftung
- Forschungskredit
- Swiss Life Jubiläumsstiftung
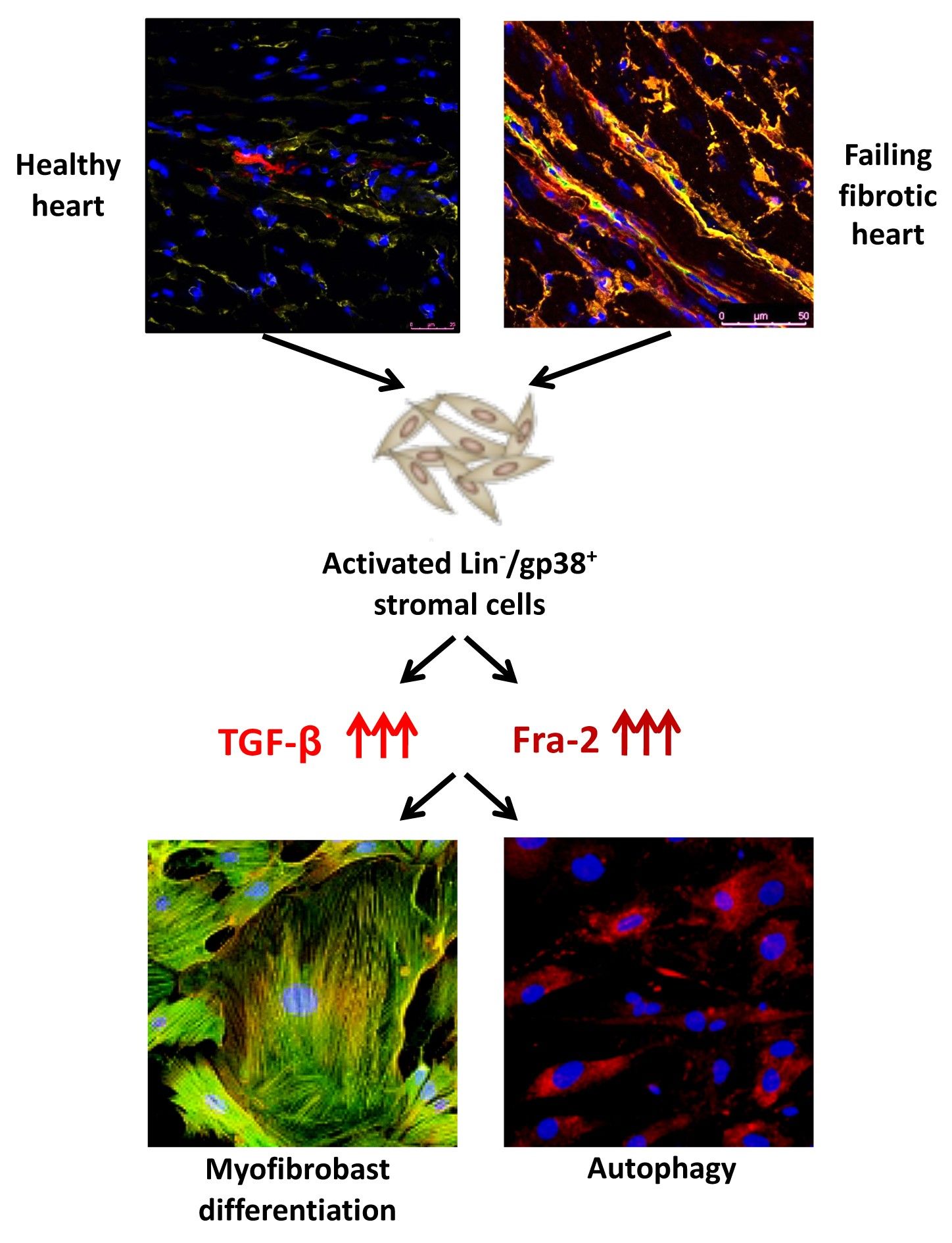
Autophagy has emerged as a central player in a multitude of immune functions and organ remodelling. Autophagy might be also regulated by Transforming Growth Factor (TGF)-β and involved in the pathogenesis of inflammatory-driven organ fibrosis, for example liver or kidney fibrosis.
We showed that the TGFβ/Fos-related antigen (Fra)-2 axis regulates the autophagy process, leading in turn to stromal-to-myofibroblast transition. Therefore, targeting autophagy might ameliorate the fibrotic process during heart remodelling in SSc.
Funding
- Bayer Grant4Target
- Kurt und Senta Hermann Stiftung
- Hermann Klaus Stiftung
- Helmut Horten Stiftung
- Baugarten Stiftung
- Hartmann Müller Stiftung
We hypothesize that specific stromal or myeloid cell populations might either serve as cellular sources of pathological myofibroblasts or activate fibrogenesis not only in myocardial but generalized in multiorgan fibrosis in SSc.
We believe that the insights from this project might contribute to general and unifying mechanistic concepts in the physiopathology of fatal multiorgan fibrosis and dysfunction not only in SSc but in other fibroproliferative disorders. To this aim we will focus on cell-to-cell interaction and on single cell level.
Funding
- Swiss National Science Foundation
- Swiss Heart Foundation
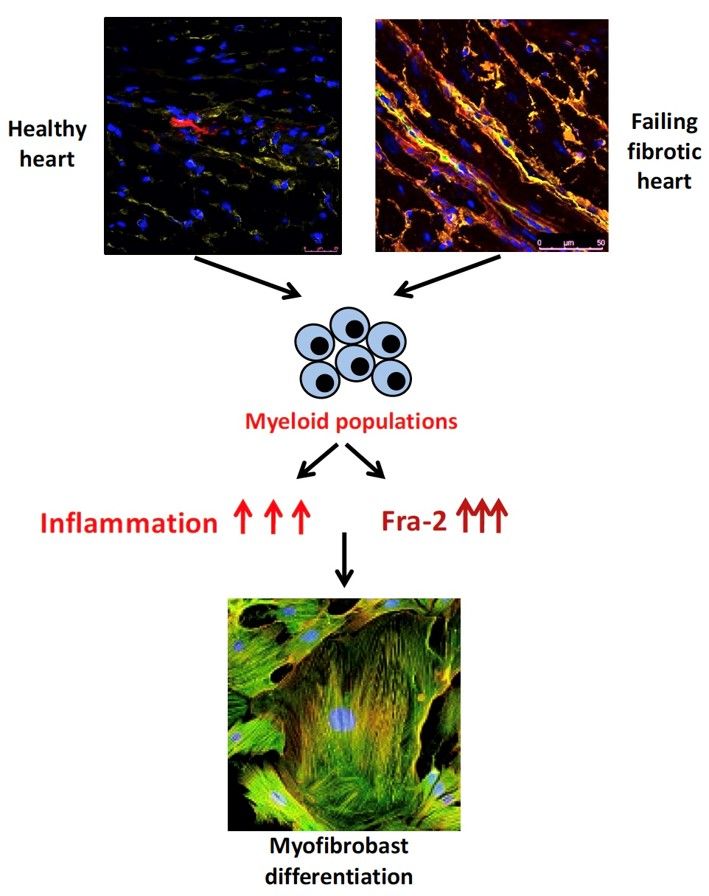
Myeloid cells function as antigen-presenting cells and as potent producers of pro-inflammatory or anti-inflammatory factors. Myeloid cells also affect the differentiation of neighbouring cells and cell progeny and modulate the local signalling milieu. In this manner, myeloid cells can actively contribute to the pathogenesis of inflammatory diseases, including SSc.
We showed the importance of Fos-related antigen (Fra)-2 in the regulation of activation stage of myeloid populations, namely monocytes and macrophages in SSc, therefore we plan further to investigate the role of myeloid populations in pathogenesis of SSc. To this aim we will focus on cell-to-cell interaction and on single cell level.
Funding
- Swiss National Science Foundation
- Swiss Heart Foundation
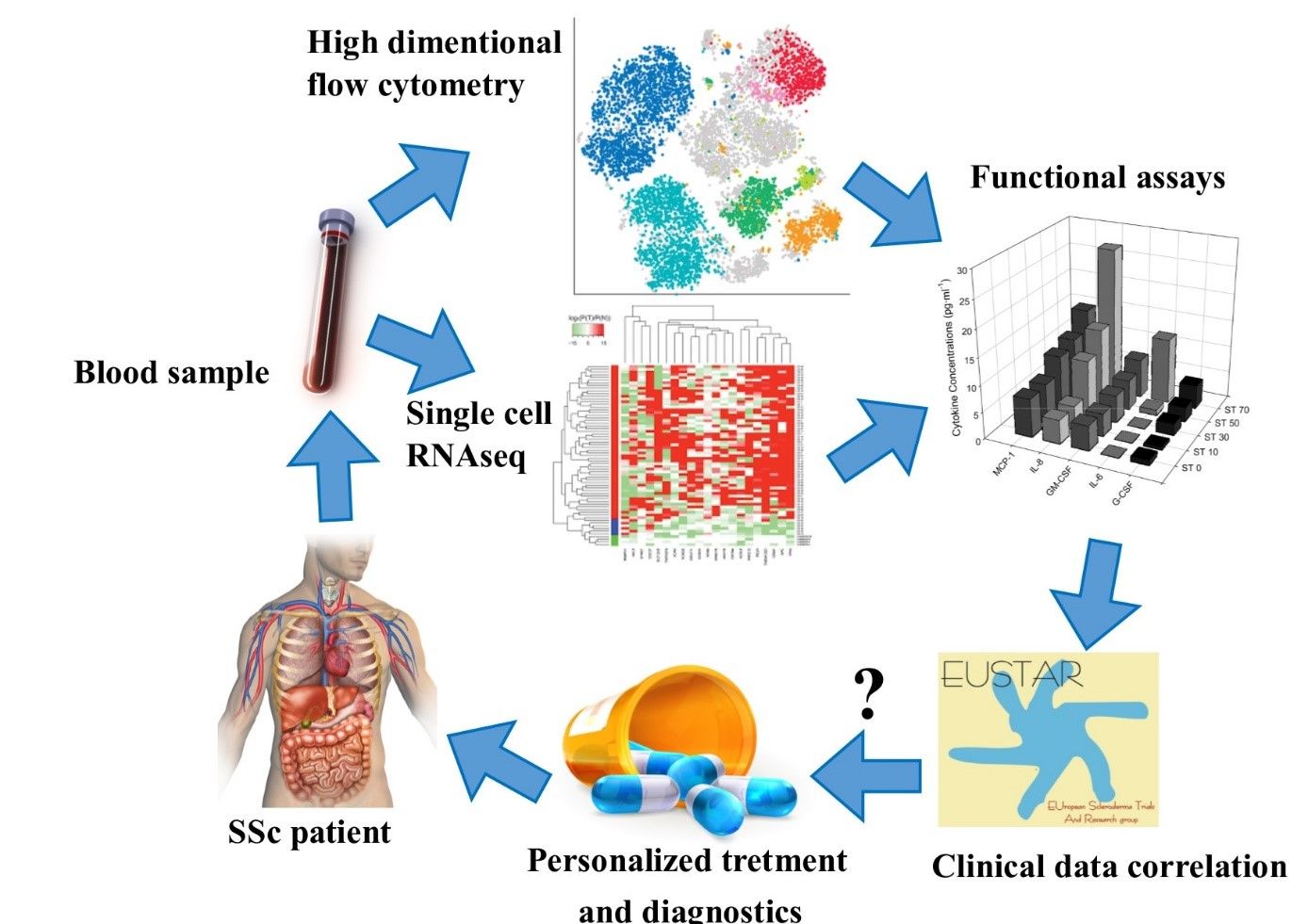
Monocytes represent a part of white blood cells, which are basically responsible for fast interventions against pathogens invading our bodies, such as bacteria, parasites and fungi. In case of body/tissue damages, they develop inflammation and mobilize other cell types in wound healing and regeneration. However, dysregulated immune system can sustain inflammation that leads to fibrosis.
We are addressing the question of what the exact role of monocytes is in the development of pathological multiorgan fibrosis in SSc patients. Additionally, we believe that detailed observation of monocyte activation state might be an indicator of disease progression and response to the treatment.
Funding
- Swiss National Science Foundation
- Swiss Heart Foundation
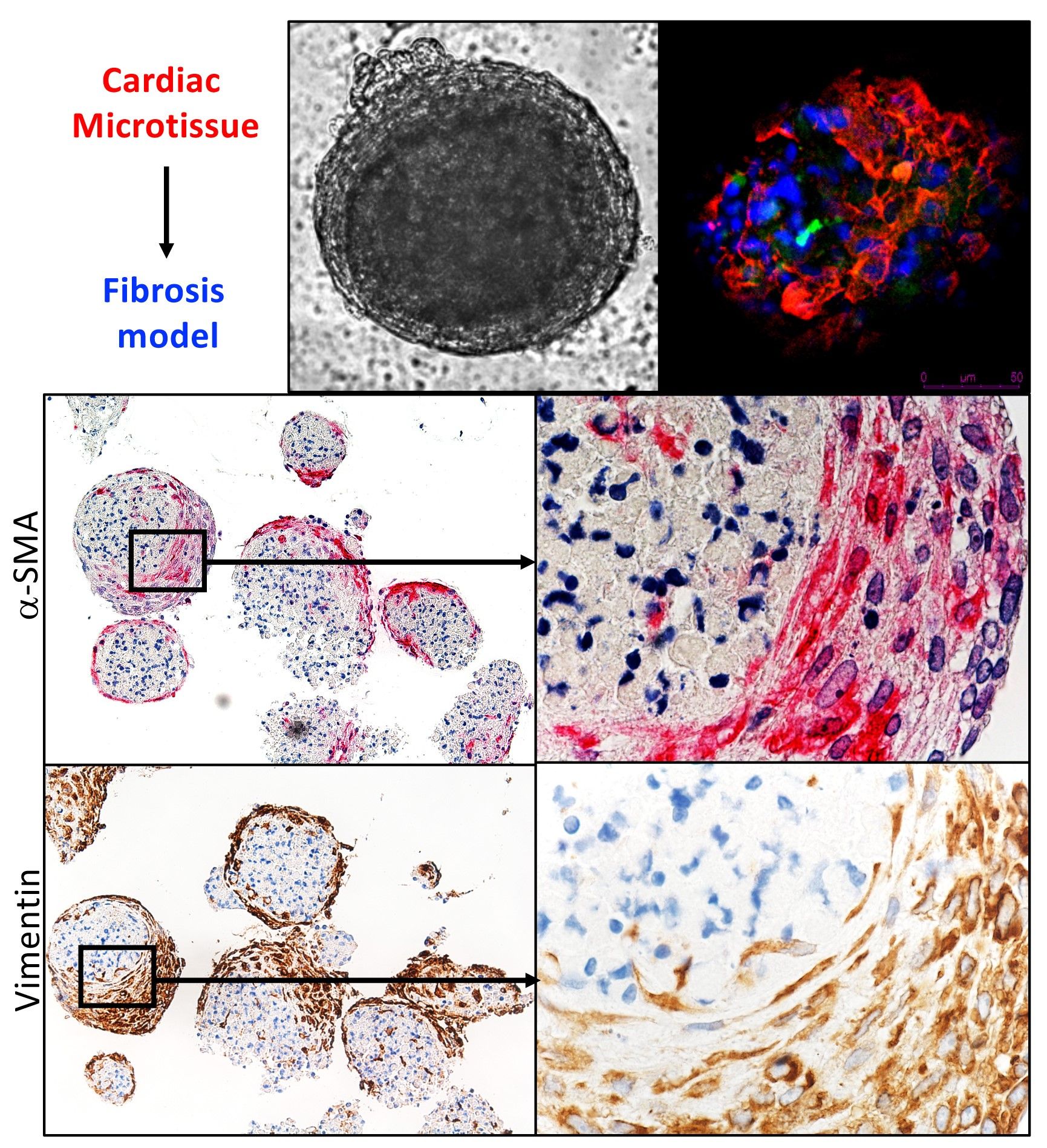
3D cardiac microtissues, composed of human-induced pluripotent stem cells and human cardiac fibroblasts, provide an organoid human-based cellular platform that is free of scaffold and necrosis, and recapitulates vital cardiac functionality mimicking contractility and electrophysiological responses.
We aim to establish 3D cardiac models of fibrosis and microvasculopathy with further evaluation of their characteristic and functions. Cell-to-cell integration in 3D in vitro model offers an excellent tool to study cell/tissue/organ physiology and pathology in SSc.
Funding
- Swiss Heart Foundation
- Gebauer Foundation
- Vontobel Foundation