Peripheral artery disease
Our aim is to explore molecular-biological mechanisms leading the development of peripheral aneurysms in the context of PAD.
The prevalence of peripheral artery disease (PAD) is 3 – 10% in the age of 50 – 60 years and increases in the elderly for over 20% [Fowkes 2013]. PAD is frequently asymptomatic, only 1 – 2% of patients develop critical limb ischaemia. These patients suffer interestingly more frequently from cardiovascular disease and/or other vascular disorders. Besides, stenotic or aneurysmal lesions within the aortic bifurcation lead in more than 95% to PAD [Fowkes 2013].
Our own results demonstrated that atherosclerotic plaques and their progression in lower extremities follow the same pattern as atherosclerotic lesions in carotid arteries [Zimmermann 2015]. Hence, vulnerable plaques can develop also in peripheral arteries and may thus lead to ischemic stroke or heart attack.
An aneurysm occurs not only in the aorta but also in the peripheral arteries, in particular in males over 70 years. In most cases (70%) femoral or iliac arteries (FA, IA) are affected. Furthermore, in 25% peripheral aneurysms are associated with aortic abdominal aortic aneurysms. These circumstances aggravate the disease progression and increase the risk of further complications.
Therefore, we focus particularly on patients with complex aneurysm with abdominal as well as femoral or iliac aneurysm. Our aim is to explore molecular-biological mechanisms leading the development of peripheral aneurysms in the context of PAD. Besides, we focus on comparing the properties of the vessel wall between AAA and FA or IA, to find potential differences.
In order to analyse the PAD tissue samples, we employ again the state-of-the-art techniques as proteomics, transcriptomics and scRNA-sequencing. Furthermore, we are using primary patient-derived smooth muscle cells (SMCs) and endothelial cells (ECs) to analyse the changes within these cells focusing in particular on epigenetics and ncRNAs.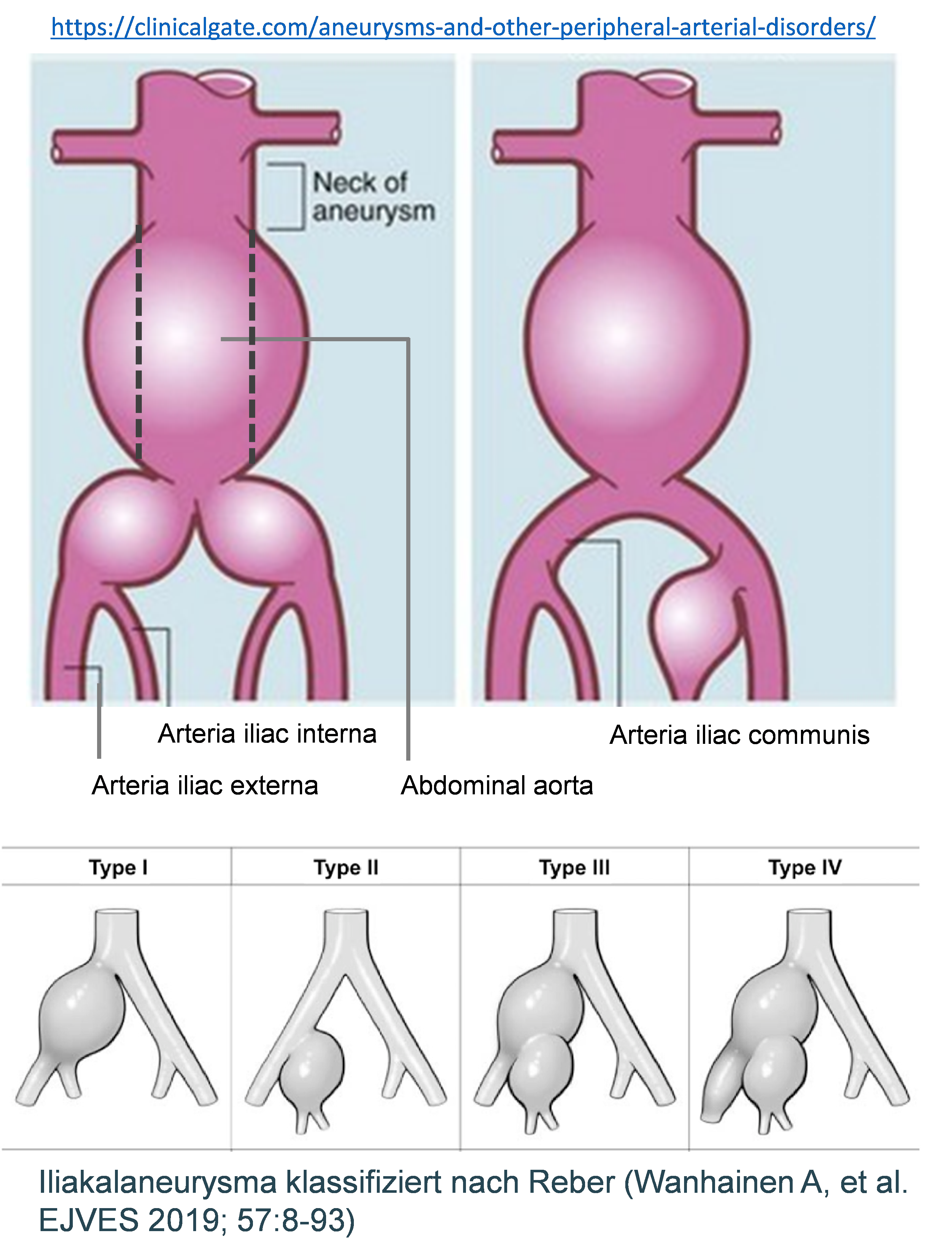
- Fowkes FG, et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet. 2013;382(9901):1329-40.
- Wildgruber M, Aschenbrenner T, Wendorff H, Czubba M, Glinzer A, Haller B, Schiemann M, Zimmermann A, Berger H, Eckstein HH, Meier R, Wohlgemuth WA, Libby P, Zernecke A. The “Intermediate” CD14++CD16+ monocyte subset increases in severe peripheral artery disease in humans. Sci Rep. 2016;6:39483.
- Rasper M, Wildgruber M, Settles M, Eckstein HH, Zimmermann A, Reeps C, Rummeny EJ, Huber AM. 3D non-contrast-enhanced ECG-gated MR angiography of the lower extremities with dual-source radiofrequency transmission at 3.0T: Intraindividual comparison with contrast-enhanced MR angiography in PAOD patients. Eur Radiol. 2016;26(9):2871-80.
- Zimmermann A, Senner S, Eckstein HH, Pelisek J. Histomorphological evaluation of atherosclerotic lesions in patients with peripheral artery occlusive disease. Adv Med Sci. 2015;60(2):236-9.
- Zimmermann A, Wendorff H, Schuster T, Auer F, Berger H, Eckstein HH. Interobserver agreement of the TASC II classification for supra- and infrainguinal lesions. Eur J Vasc Endovasc Surg. 2010;39:586-90.